What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 23 dezembro 2024

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

5 Steps to More Efficient Computer System Validation

Computerized system validation (CSV) in Pharmaceutical industry l 25 Interview Question
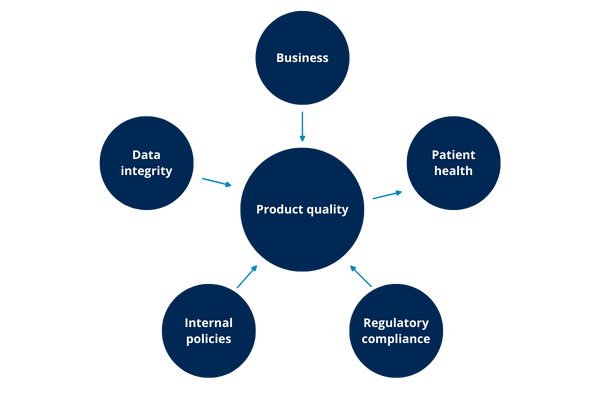
A Complete Guide to Computer System Validation (CSV): What is it and why do we need it?

What You Should Know About CSV in Pharma

Computer System Validation - NNIT

The Complete Guide to Computer System Validation: IQ, OQ, PQ, – Blue Mountain

Understanding FDA's CSA Guidance in the Context of Current Regulations and GAMP® American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
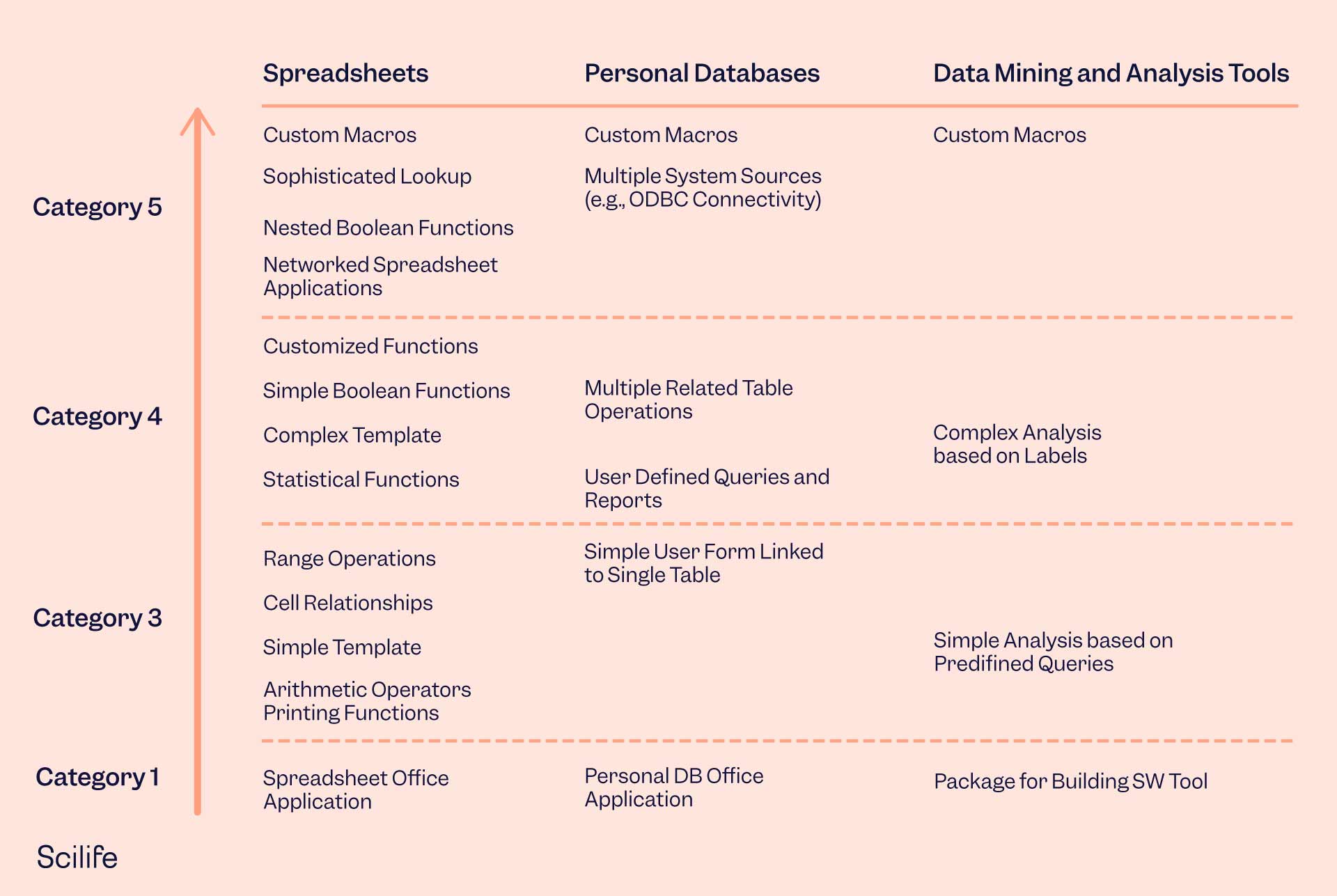
CSV vs. CSA: What Are the Main Differences?
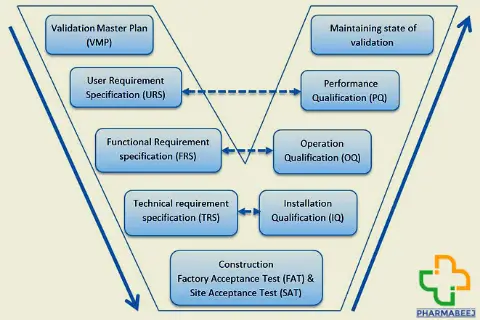
Computer System Validation (CSV) As Per FDA - Pharmabeej
CSA – fad or trend? - Körber Pharma
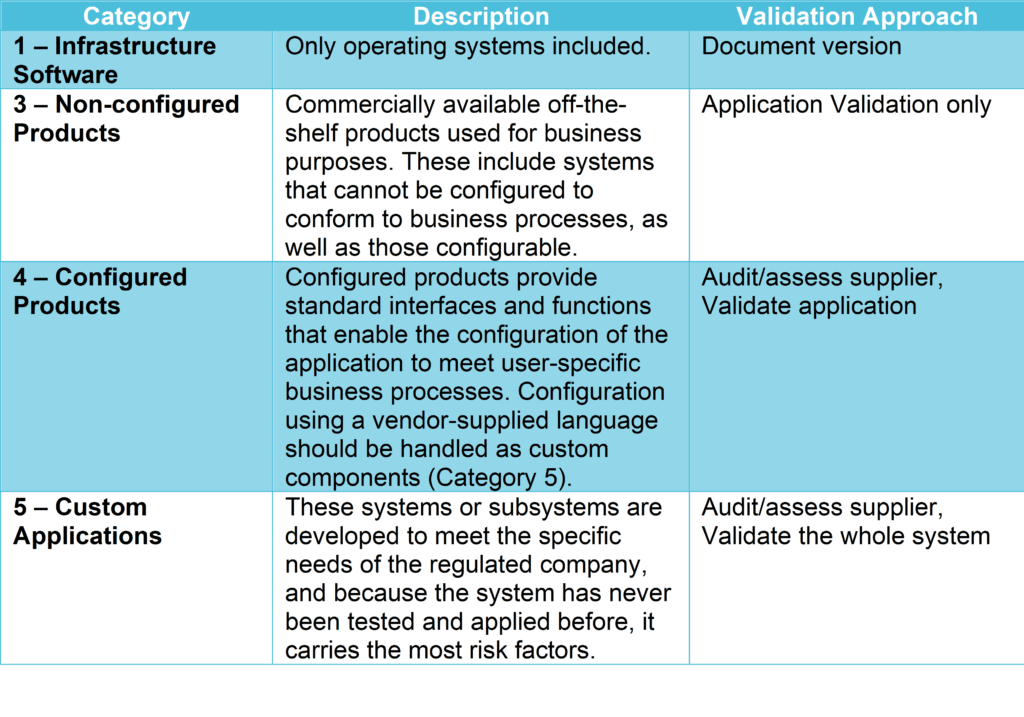
Risk-Based Computerized System Validation (CSV) and Computer Software Assurance (CSA) - Old Wine in a New Bottle? - Kvalito

What is Computer System Validation?
Recomendado para você
-
 COLÉGIO SÃO VICENTE DE PAULO SÃO LUÍS / MA23 dezembro 2024
COLÉGIO SÃO VICENTE DE PAULO SÃO LUÍS / MA23 dezembro 2024 -
 Save on Columbus Salame Sopressata Chub Order Online Delivery23 dezembro 2024
Save on Columbus Salame Sopressata Chub Order Online Delivery23 dezembro 2024 -
 Pacific Merchant Shipping Association23 dezembro 2024
Pacific Merchant Shipping Association23 dezembro 2024 -
 Información de tráfico en tiempo real para llegar a Colégio São23 dezembro 2024
Información de tráfico en tiempo real para llegar a Colégio São23 dezembro 2024 -
 ASCM San Fernando Valley Chapter CPIM CSCP CLTD23 dezembro 2024
ASCM San Fernando Valley Chapter CPIM CSCP CLTD23 dezembro 2024 -
 Напътствия до Colégio São Vicente de Paulo, Av. São Marçal, 20423 dezembro 2024
Напътствия до Colégio São Vicente de Paulo, Av. São Marçal, 20423 dezembro 2024 -
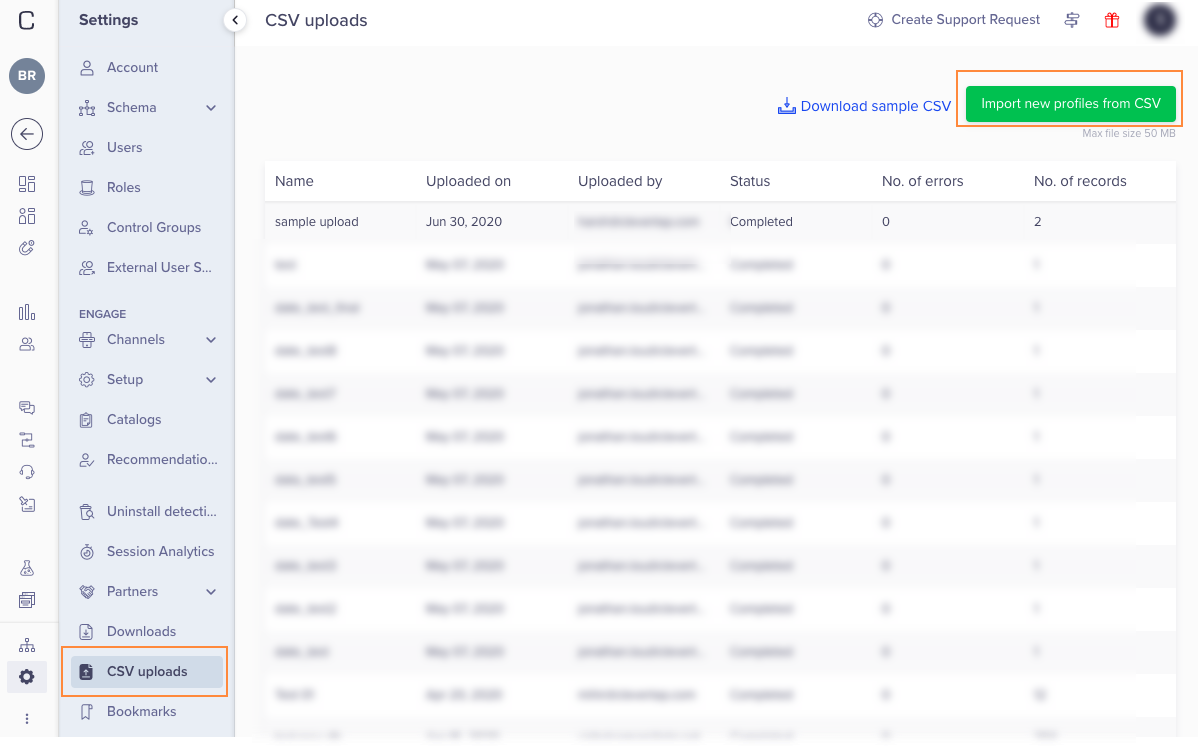 CSV Upload23 dezembro 2024
CSV Upload23 dezembro 2024 -
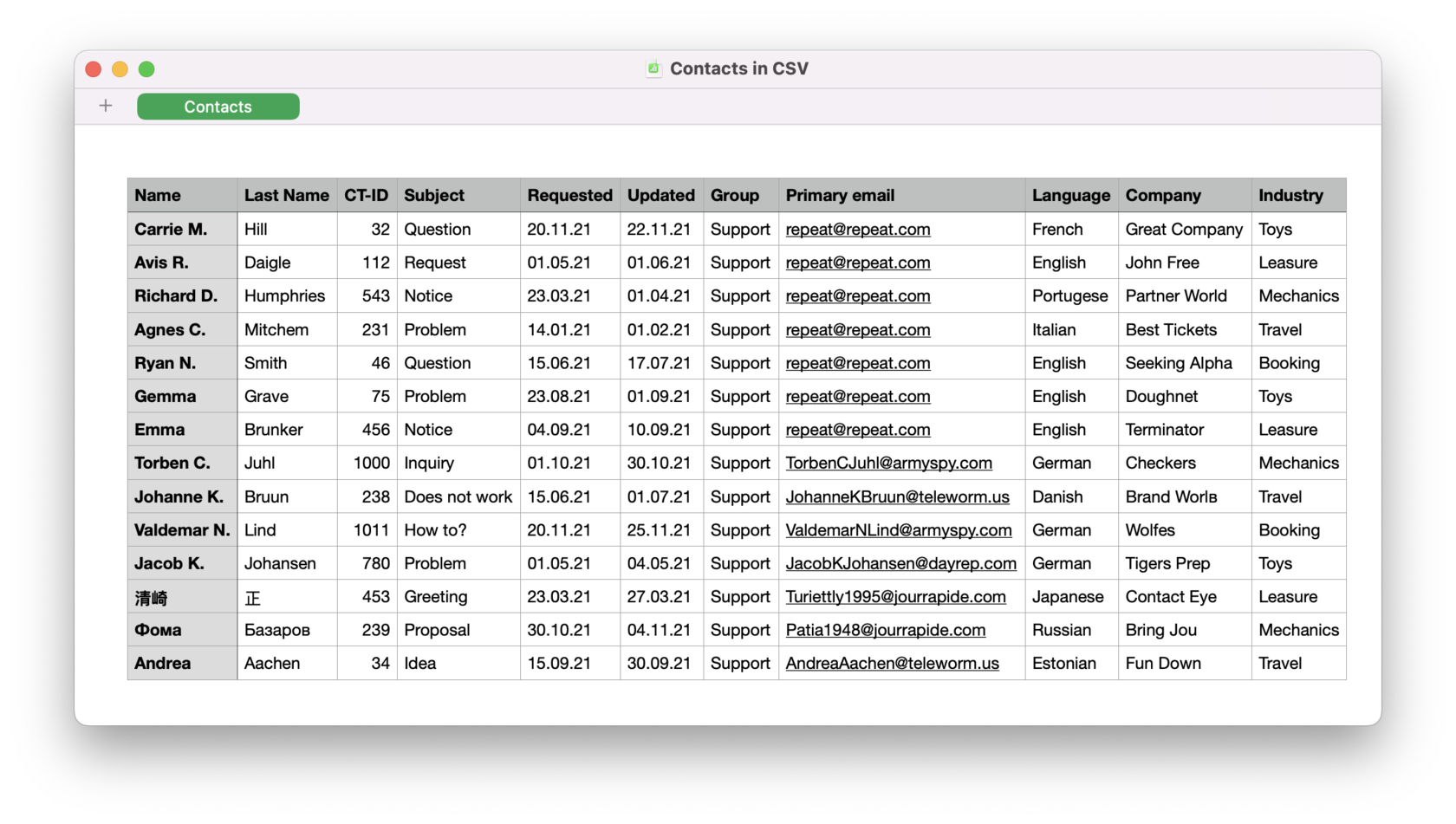 What is CSV?23 dezembro 2024
What is CSV?23 dezembro 2024 -
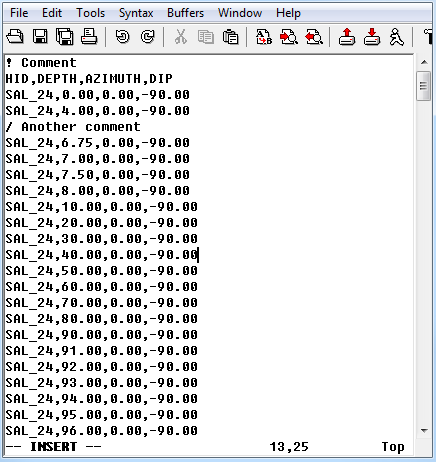 5.1.2. CSV file format — GIFtoolsCookbook 1.0 documentation23 dezembro 2024
5.1.2. CSV file format — GIFtoolsCookbook 1.0 documentation23 dezembro 2024 -
 2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars23 dezembro 2024
2017 Audi A3 2.0 TDI S LINE 2.0 Diesel Manual - £15250 - PMA Cars23 dezembro 2024
você pode gostar
-
Industrial Engines, Cat23 dezembro 2024
-
 UUB MUI by adb3388 on DeviantArt23 dezembro 2024
UUB MUI by adb3388 on DeviantArt23 dezembro 2024 -
 Ice Scream 7 full gameplay23 dezembro 2024
Ice Scream 7 full gameplay23 dezembro 2024 -
Erste Bank Open (ATP Vienna)23 dezembro 2024
-
 Ferrari disponibiliza jogo de corrida gratuito23 dezembro 2024
Ferrari disponibiliza jogo de corrida gratuito23 dezembro 2024 -
Big Floppa, el gato salvaje que se convirtió en un meme En una casa hogareña se encuentra la mascota más impresionante. Es Gosha, un caracal domesticado. Juega y comparte con otros23 dezembro 2024
-
 STL file Suporte Dock Station Apple Watch Coruja Hedwig Harry Potter・3D print design to download・Cults23 dezembro 2024
STL file Suporte Dock Station Apple Watch Coruja Hedwig Harry Potter・3D print design to download・Cults23 dezembro 2024 -
 Solitaire Games Play Free Online at Solitaire 36523 dezembro 2024
Solitaire Games Play Free Online at Solitaire 36523 dezembro 2024 -
 quanto e : exemplo , 35 minutos e quntos segundos ? tipo isso tipo isso 23 dezembro 2024
quanto e : exemplo , 35 minutos e quntos segundos ? tipo isso tipo isso 23 dezembro 2024 -
música só para jogador caro|Pesquisa do TikTok23 dezembro 2024



